The Structure of 3-propylpentane Is Best Described as Brainly
It can form covalent bonds. 2 carbon is the one which is attached to 2 carbon atom.

The Structure Of 3 Propylpentane Is Best Described As A A Three Carbon Chain With A Three Carbon Brainly Com
45-dimethylpentane is incorrect not only is it not using the lowest constituents numbers in the name IE.
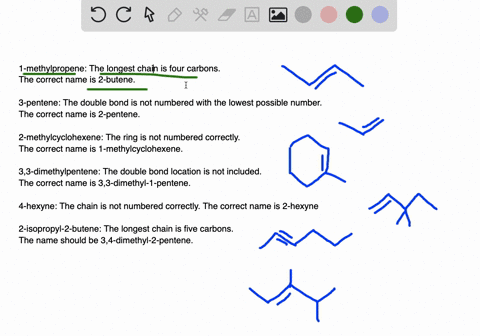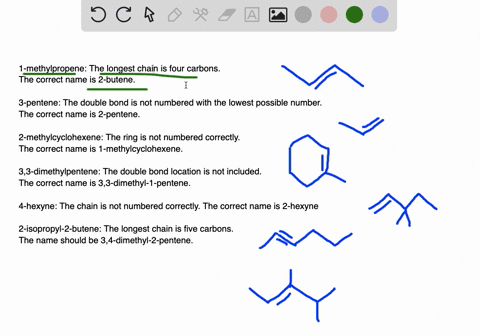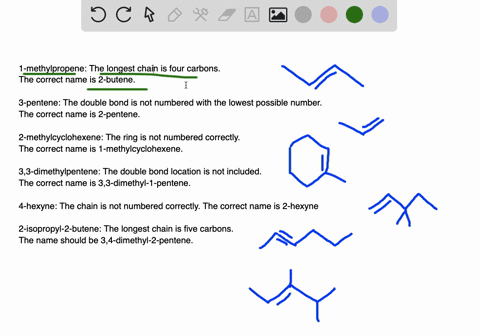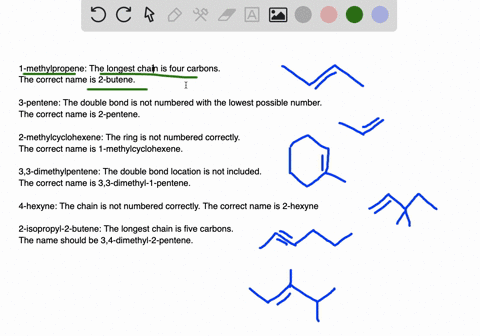
. A five-carbon chain with a three-carbon branch on the third carbon in the chain c. Because we have to choose always the longest chain. A Propanedial B 4-Phenylbutanal C S -3-Phenylbutanal D 3355.
Name the following compound 3 - ethyl -3 - methyihexane 3-ethy-3- propyibutane nonane 3-methyl-3-propylpentane 2 - ethylheptane Which of the following compounds exhibits geometric isomerism. A substitution reaction can best be described as a reaction in which Question 26 options. The propoxy group is a 3-carbon chain with an O atom at C-1.
A five-carbon ring with a three-carbon branch on the third carbon in the ring d. Single-stranded chains in compact stem-and-loop structures. So that is a seven carbon alkane.
Attach an ethoxy group to C-2 of a butane chain. National Institutes of Health. National Center for Biotechnology Information.
So when do you see something like this you might immediately say-- and the way we drew it was actually correct it just wouldnt be called 2-Propylheptane. For the methyl cation CH3 the geometry H-C-H bond angle and hybridization of the carbon atom is best described as. A two reactants combine to form one new product with no extra atoms.
What is the approximate bond angle around the carbon in formaldehyde a molecule with the formula H2CO. B For reversible reactions product 2 will dominate at equilibrium. Let me show you what Im talking about.
The structure of 3-propylpentane is best described as a. 1 carbon is the one which is attached to 1 carbon atom. Other relevant information includes the following.
A single carbon atom always bonds with four other atoms Wrong because Carbon atoms form four bonds but that does not necessarily mean they will always bond with four atoms. Draw a chain of five carbon atoms and attach the 1-propoxy group to C-1 of the chain. Trigonal planar 120 degsp2.
Pentane is a chain of 5 carbon atoms. A three-carbon chain with a three-carbon branch on the third carbon in the chain b. Give the IUPAC name for each of the following.
3-propylpentane-24-dione Pre-Registration process. In case of consumer and professional uses. 3-Propylhexane-23-diol C9H20O2 CID 458089 - structure chemical names physical and chemical properties classification patents literature biological.
So you say heptane. Neither reflect other factors that affect the susceptibility of the effects described such as duration of exposure or substance concentration eg. Problem Set Organic Answer Key - Spaces Login - Imperial.
Double-stranded helices Extended single-stranded chains Single-stranded chains in compact stem-and-loop structures Small circular chains. A For irreversible reactions product 1 will dominate. 3-Methyl-3-propylpentane-15-diol C9H20O2 CID 13928740 - structure chemical names physical and chemical properties classification patents literature.
No such structure exist. 2-Ethyl-3-propylpentane-15-diol C10H22O2 - PubChem. National Library of Medicine.
What statement is true of the following energy diagram. B a hydrocarbon reacts with oxygen to produce CO2 H2O and energy. 3 carbon is the one which is attached to 3 carbon atom.
The name of the compound is different if you write 2-propyl pentane. It has 1. For example in ethylene H2CCH2 each carbon atom is bound to 3 other atoms since there is a double bond between the two carbon atoms.
No double bond so one two three four five six seven. This problem has been solved. 2-ethyl-3-propylpentane 2 3-diethylhexane 4-ethyl-5-methylheptane 4-methyl-3-ethyl heptane 4-ethyl-3-methyl heptane Question.
Answer3-Methyl-3-propylpentane-15-diol C9H20O2 CID 13928740 - structure chemical names physical. ORGANIC CHEMISTRY WOORKSHEET ON NOMENCLATURE Chemistry 112 I. A three carbon chain with a five-carbon.
AnswerPlease check attachment ExplanationIn this question we are to draw the structure for each of the compounds belowPlease check attachment for the diagra kkfinney66571 kkfinney66571 09212020 Chemistry College Draw the structure for each compound below_____. Using 12 instead of 45 it is impossible to have a methyl constituent on the first or last carbon because that would just mean the compound is not a pentane but in fact a hexane thus that name should be 2-metyhlhexane. Department of Health and Human Services.
C a single reactant undergoes reorganization of its chemical bonds producing an isomer of the reactant. 4 carbon is the one which is attached to 4 carbon atom. 8600 Rockville Pike Bethesda MD 20894 USA.
What is the expected bond angle for the C-C-O bonds in the molecule below. CBr_2 CHBr CH_3 - CH CH CH-CH_3 CH_2 CH-CH_3 CCII_2 CF_2 All of the above exhibit geometric isomerism. Structure of 223-trimethylhexane is as follows.
Chemistry questions and answers. A 3-sec-pentylpentane B 5-ethyl-4-methylheptane C 3-sec-butylhexane D 3-ethyl-4-methylheptane E 3-ethyl-4-propylpentane Page 3 8. Which statement best describes the typical structure of RNA as found in cells.

Solved Interpret Chemical Structures Why Is The Name 3 Butylpentane Incorrect Based On This Name Write The Structural Formula For The Compound What Is The Correct Iupac Name For 3 Butylpentane

The Structure Of 3 Propylpentane Is Best Described As A A Three Carbon Chain With A Three Carbon Brainly Com

The Structure Of 3 Propylpentane Is Best Described As A A Three Carbon Chain With A Three Carbon Brainly Com
Comments
Post a Comment